
Exhibit 99.2

CBD - BASED PHARMACEUTICAL & CONSUMER PRODUCTS Corporate Presentation – August 4, 2018 Symbol: CVSI

This presentation may contain certain forward - looking statements and information, as defined within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and is subject to the Safe Harbor created by those sections. This material contains statements about expected future events and/or financial results that are forward - looking in nature and subject to risks and uncertainties. Such forward - looking statements by definition involve risks and uncertainties. This Overview does not constitute an offer to sell or a solicitation of an offer to buy any securities of CV Sciences, Inc. The company is not soliciting any investment from this presentation or event attendees. Offers to sell or solicitations of offers to buy securities of the company will be made pursuant to the registration requirements of the Securities Act of 1933, or regulations of the Securities and Exchange Commission, and under relevant state securities laws; or in accordance with lawful exemptions from registration requirements under applicable federal and state securities laws and regulations. SAFE HARBOR & DISCLAIMER 2 SYMBOL: CVSI

CV Sciences, Inc. ▪ Operations in: - San Diego, CA - Lexington, KY - Las Vegas, NV ▪ Founded in 2012 ▪ 70+ employees ▪ Two operating divisions: 1. Consumer products 2. Drug development ▪ Active ingredient - “Cannabidiol” (CBD) CORPORATE OVERVIEW 3 SYMBOL: CVSI
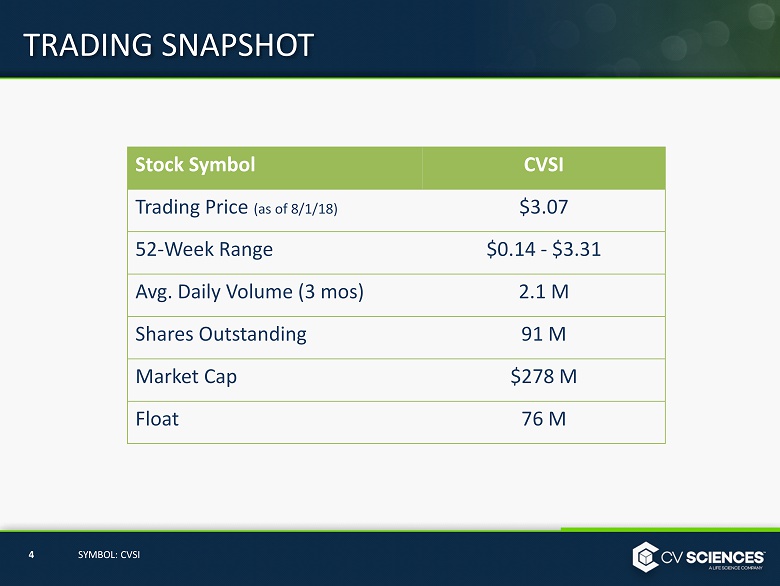
Stock Symbol CVSI Trading Price (as of 8/1/18) $3.07 52 - Week Range $0.14 - $3.31 Avg. Daily Volume (3 mos ) 2.1 M Shares Outstanding 91 M Market Cap $278 M Float 76 M TRADING SNAPSHOT 4 SYMBOL: CVSI

CONSUMER PRODUCTS DIVISION 5 SYMBOL: CVSI

CONSUMER PRODUCTS OVERVIEW Overview ▪ 50+ SKUs of branded products ▪ Sales channels include: ▪ 1968 retail locations nationwide ▪ 1000+ doctor offices nationwide ▪ Strong wholesale presence ▪ Growing ecommerce channel ▪ #1 Selling Hemp - based CBD Products* #2 Company in the Natural Products Retailer* *According to SPINS Scan Data ▪ Category sales forecast for hemp - based CBD products ≈ $1.6 Billion by 2021 6 SYMBOL: CVSI

CONSUMER PRODUCTS Raw Formula Total Plant Complex Gold Formula Branded Product: PlusCBD Oil™ 50+ SKUs 7 SYMBOL: CVSI

RETAIL STORE PLACEMENT 8 SYMBOL: CVSI
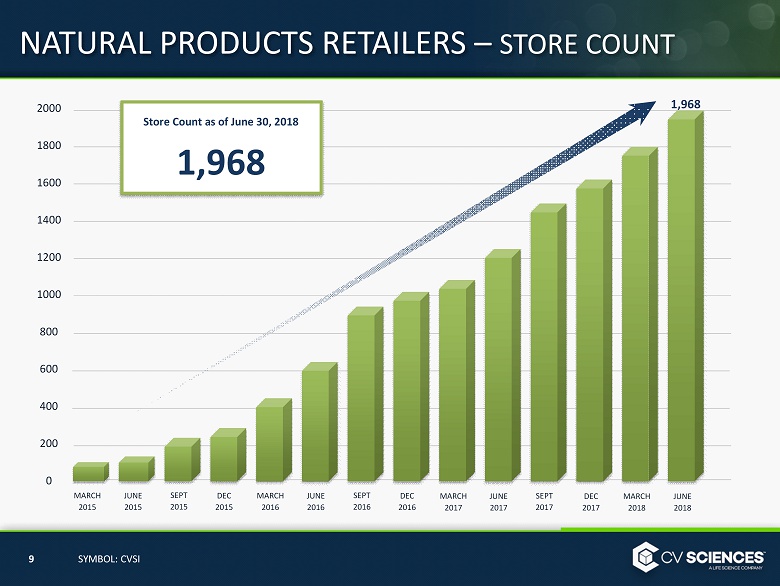
NATURAL PRODUCTS RETAILERS – STORE COUNT 20 00 1 8 00 1600 1400 1200 1000 800 600 400 200 0 MARCH 2015 9 SYMBOL: CVSI JUNE 2015 SEPT 2015 DEC 2015 MARCH 2016 JUNE 2016 SEPT 2016 DEC 2016 MARCH 2017 JUNE 2017 SEPT 2017 DEC 2017 MARCH 2018 JUNE 2018 Store Count as of June 30, 2018 1,968 1,968
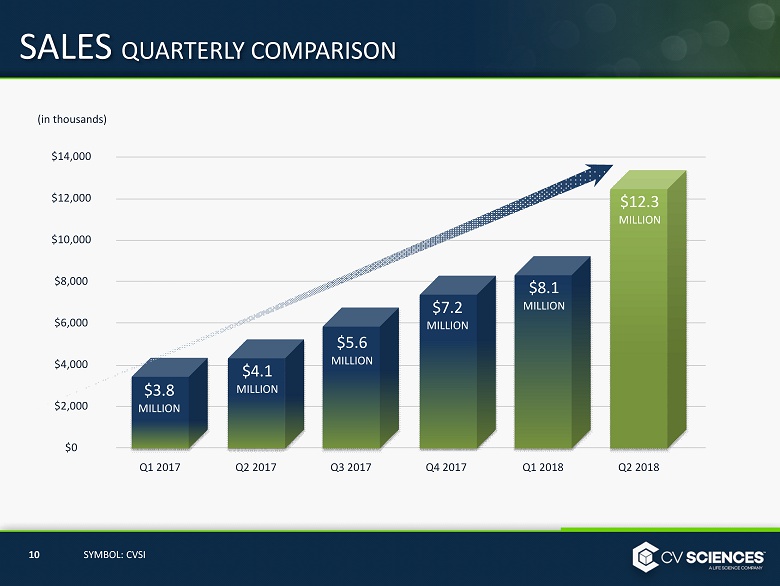
SALES QUARTERLY COMPARISON $14,000 $12,000 $ 10,000 $8,000 $6,000 $4,000 $2,000 $0 Q1 2017 Q1 2018 Q2 2017 Q3 2017 Q4 2017 Q 2 2018 10 SYMBOL: CVSI (in thousands) $3.8 MILLION $4.1 MILLION $7.2 MILLION $8.1 MILLION $ 5.6 MILLION $ 12.3 MILLION
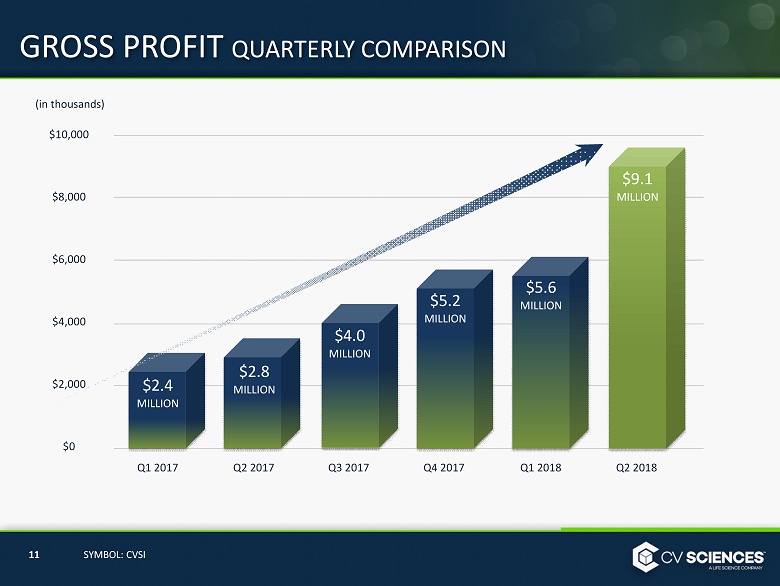
GROSS PROFIT QUARTERLY COMPARISON $ 10,000 $8,000 $6,000 $4,000 $2,000 $0 Q1 2017 Q1 2018 Q2 2017 Q3 2017 Q4 2017 Q 2 2018 11 SYMBOL: CVSI (in thousands) $ 2.4 MILLION $ 2.8 MILLION $ 5 .2 MILLION $ 5.6 MILLION $ 4.0 MILLION $ 9.1 MILLION
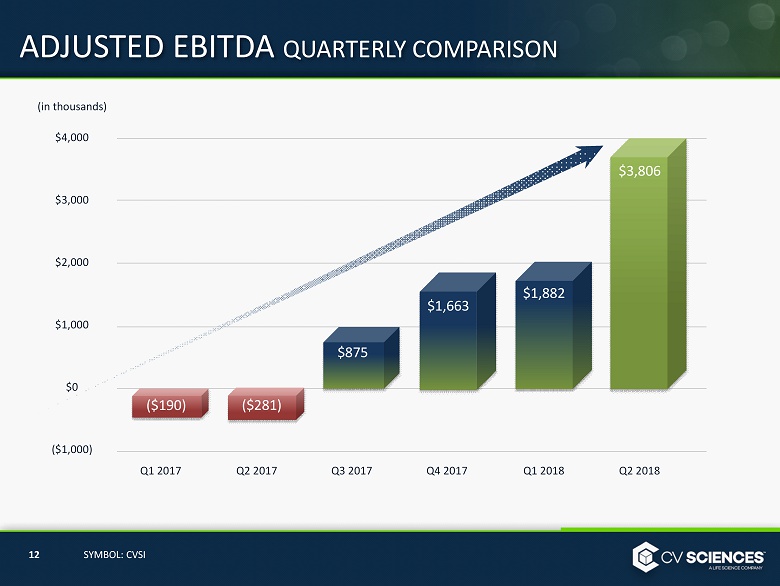
ADJUSTED EBITDA QUARTERLY COMPARISON $ 4,000 $ 3 ,000 $ 2 ,000 $ 1 ,000 $0 ($1,000) Q1 2017 Q1 2018 ($190) Q2 2017 Q3 2017 Q4 2017 Q 2 2018 ($281) 12 SYMBOL: CVSI (in thousands) $1,663 $1,882 $875 $3,806
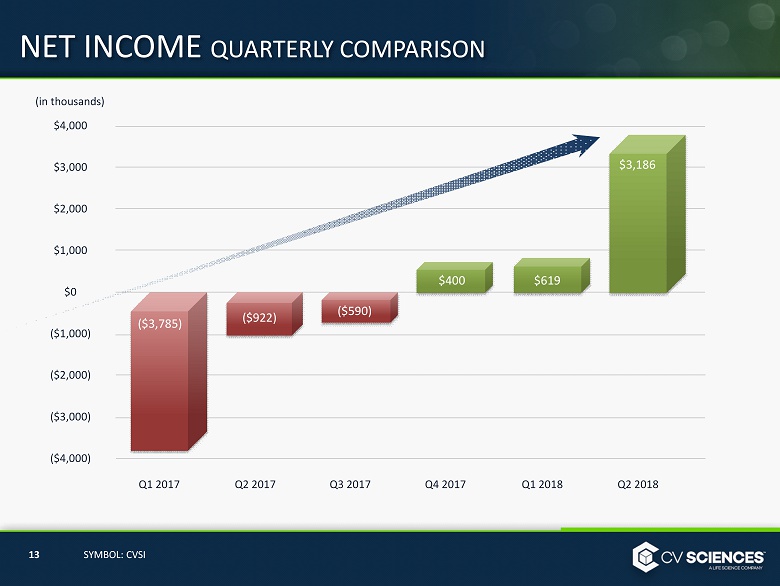
NET INCOME QUARTERLY COMPARISON $ 4,000 $ 3 ,000 $ 2 ,000 $ 1 ,000 $0 ($1,000) ($2,000) ($3,000) ($4,000) Q1 2017 Q1 2018 $400 Q2 2017 Q3 2017 Q4 2017 $619 $3,186 Q 2 2018 ($922) ($590) 13 SYMBOL: CVSI (in thousands) ($3,785)

SPINS DATA SPINS is the leading provider of analytics reporting for the Natural, Organic and Specialty Products Industry. 14 SYMBOL: CVSI PlusCBD Oil™ is Ranked the #1 Selling Hemp CBD Product According to SPINS Scan Data.
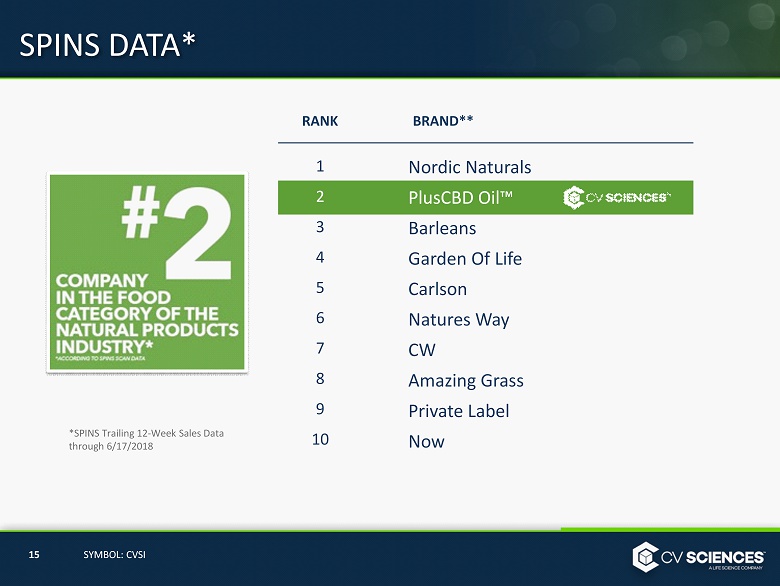
SPINS DATA* *SPINS Trailing 12 - Week Sales Data through 6 / 17 /2018 Nordic Naturals PlusCBD Oil ™ Barleans Garden Of Life Carlson Natures Way CW Amazing Grass Private Label Now 1 2 3 4 5 6 7 8 9 10 RANK BRAND** 15 SYMBOL: CVSI

PRODUCT SAFETY 16 SYMBOL: CVSI • Dietary Supplement Health and Education Act of 1994 “DSHEA” • Amended the Federal Food, Drug, and Cosmetic Act - to establish standards for dietary supplements • Generally Recognized as Safe – “GRAS”

PRODUCT SAFETY 17 SYMBOL: CVSI Published Paper

PRODUCT SAFETY 18 SYMBOL: CVSI IMPACT: GRAS self - affirmation has opened the market for… • Omega - 3 • CoQ10 • Collagen • Krill Oil
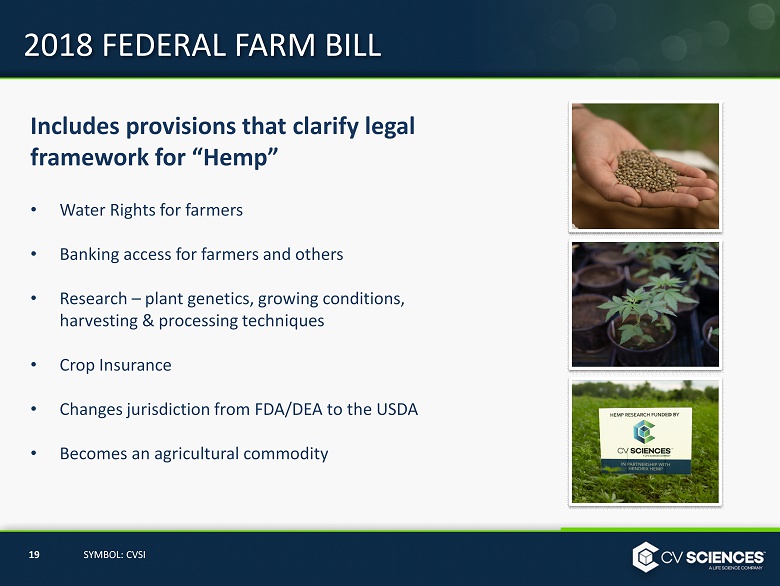
2018 FEDERAL FARM BILL Includes provisions that clarify legal framework for “Hemp” • Water Rights for farmers • Banking access for farmers and others • Research – plant genetics, growing conditions, harvesting & processing techniques • Crop Insurance • Changes jurisdiction from FDA/DEA to the USDA • Becomes an agricultural commodity 19 SYMBOL: CVSI

WALL STREET – GETTING INVOLVED Estimated legal cannabis sales of $50 billion by 2026. Currently, sales are $6 billion. 20 SYMBOL: CVSI
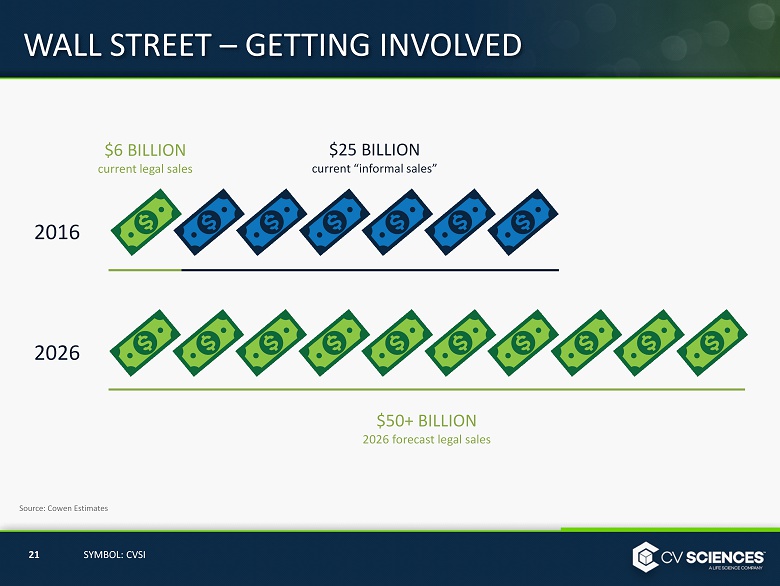
WALL STREET – GETTING INVOLVED $6 BILLION current legal sales 21 SYMBOL: CVSI Source: Cowen Estimates 2016 2026 $25 BILLION current “informal sales” $50+ BILLION 2026 forecast legal sales
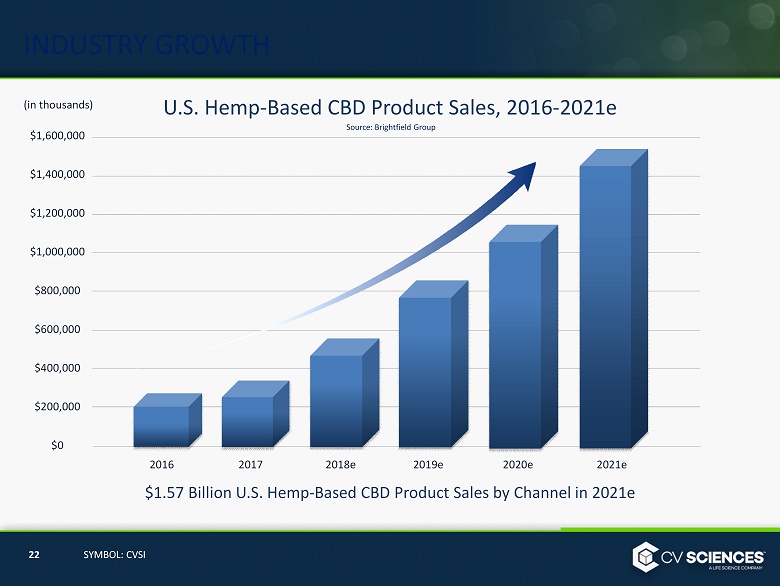
INDUSTRY GROWTH 22 SYMBOL: CVSI Source: Brightfield Group $1,600,000 $1,400,000 $1,200,000 $1,000,000 $800,000 $600,000 $400,000 $200,000 $0 2018e 2016 2017 2019e 2020e U.S. Hemp - Based CBD Product Sales, 2016 - 2021e $1.57 Billion U.S. Hemp - Based CBD Product Sales by Channel in 2021e 2021e (in thousands)

DRUG DEVELOPMENT DIVISION 23 SYMBOL: CVSI
DRUG DEVELOPMENT PROGRAM Overview ▪ CVSI - 007 – lead drug candidate ▪ Cannabidiol (CBD) and nicotine combination ▪ Medical indication – to support cessation of smokeless tobacco use and addiction ▪ Proprietary technology (patent pending) ▪ Seeking 505(b)(2) drug approval pathway 24 SYMBOL: CVSI
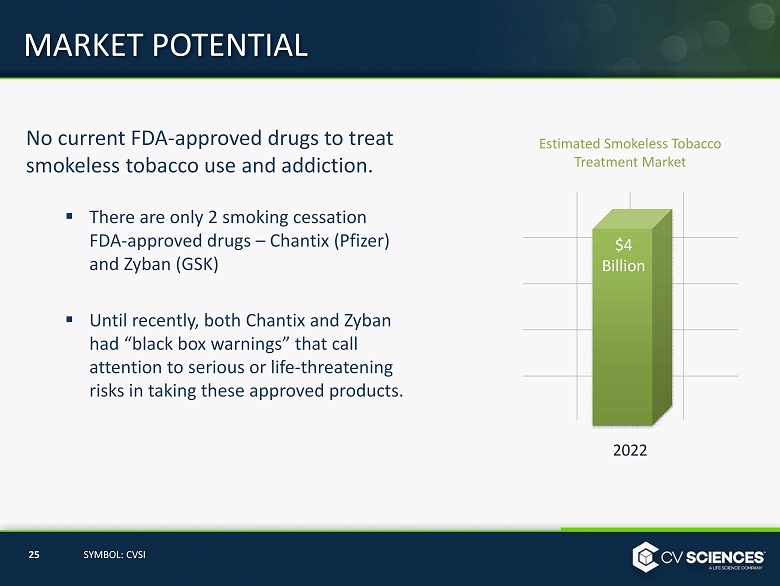
No current FDA - approved drugs to treat smokeless tobacco use and addiction. ▪ There are only 2 smoking cessation FDA - approved drugs – Chantix (Pfizer) and Zyban (GSK) ▪ Until recently, both Chantix and Zyban had “black box warnings” that call attention to serious or life - threatening risks in taking these approved products. MARKET POTENTIAL 25 SYMBOL: CVSI 2022 Estimated Smokeless Tobacco Treatment Market $4 Billion

2016 REVENUE: Approximately $1 billion CURRENT FDA - APPROVED TREATMENTS 26 SYMBOL: CVSI

Urgent Unmet Medical Need ▪ Smokeless tobacco is carcinogenic (risk of esophageal, oral and pancreatic cancers). ▪ Smokeless tobacco is one of the most addictive and potent ways of consuming nicotine (an average - size dip in the mouth for just 30 minutes can deliver as much nicotine as smoking three cigarettes). ▪ Smokeless tobacco is strongly addictive (equal to cocaine and heroin). ▪ Smokeless tobacco is an epidemic as recognized by the surgeon general. There are currently NO FDA - approved drugs to support cessation of smokeless tobacco use and addiction. UNMET MEDICAL NEED 27 SYMBOL: CVSI

Selected Epidemic Statistics ▪ Serious impact on youth: 5.8% of males 12 - 17 years of age reported use of smokeless tobacco. ▪ 49% of all military personnel used a tobacco product in the last 12 months. ▪ 12.8% of all military personnel used smokeless tobacco in the last month. EPIDEMIC: SMOKELESS TOBACCO USE 28 SYMBOL: CVSI
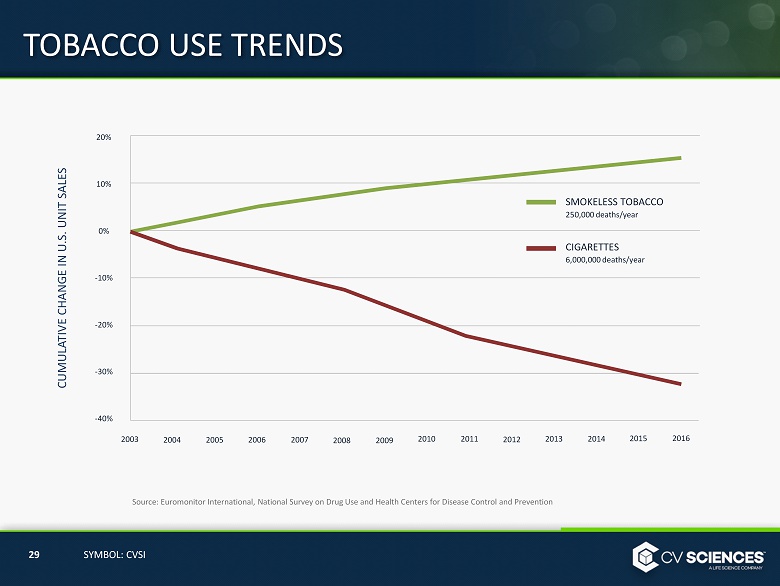
Source: Euromonitor International, National Survey on Drug Use and Health Centers for Disease Control and Prevention TOBACCO USE TRENDS 20% 10% 0% - 10% - 20% - 30% - 40% 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 CUMULATIVE CHANGE IN U.S. UNIT SALES SMOKELESS TOBACCO 250,000 deaths/year CIGARETTES 6,000,000 deaths/year 29 SYMBOL: CVSI
Financial Impact ▪ $300 Billion+ per year Direct medical care and lost productivity Sources: CDC and Tobacco Use and the Military NICOTINE ADDICTION – FINANCIAL IMPACT 30 SYMBOL: CVSI
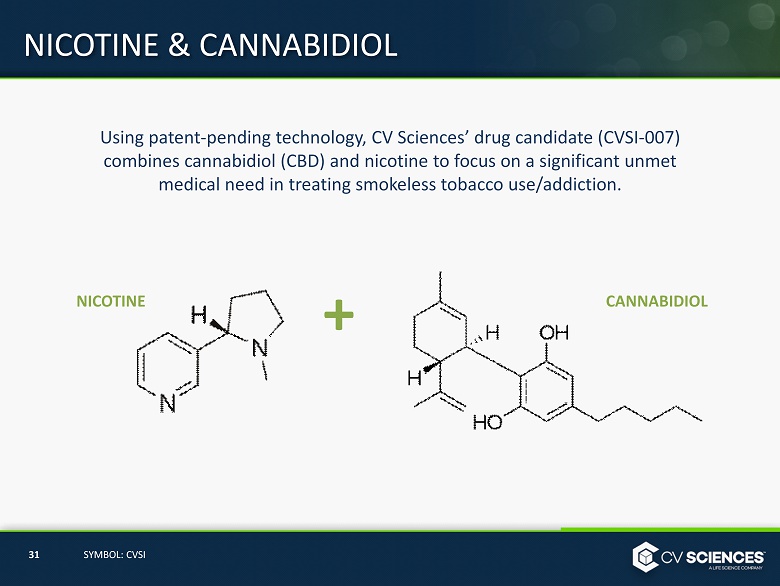
Using patent - pending technology, CV Sciences’ drug candidate (CVSI - 007) combines cannabidiol (CBD) and nicotine to focus on a significant unmet medical need in treating smokeless tobacco use/addiction. NICOTINE CANNABIDIOL + NICOTINE & CANNABIDIOL 31 SYMBOL: CVSI

Overview ▪ Physical Dependence relaxant, stimulant, increased heart rate and blood pressure, decreased appetite ▪ Emotional Dependence “oral stimulation” of tobacco product used ▪ Mental Dependence nicotine is a mild “antidepressant” NICOTINE USE & ADDICTION 32 SYMBOL: CVSI

NICOTINE PK/PD - ANTIDEPRESSANT Nicotine reaches the brain in 7 - 10 seconds Nicotine is a mild antidepressant ▪ Mechanism of Action: monoamine oxidase (“MAO”) inhibitor ▪ MAO is an enzyme that breaks down dopamine and other “feel good” neurotransmitters that produce pleasure and reward ▪ Lower levels of MAO result in higher dopamine levels Nicotine half - life is approximately 1 hour ▪ Nicotine’s PK/PD properties enhance its abuse potential with rapid distribution to the brain, drug levels peaking very quickly, and the acute effects dissipating quickly, requiring continued dosing. 33 SYMBOL: CVSI
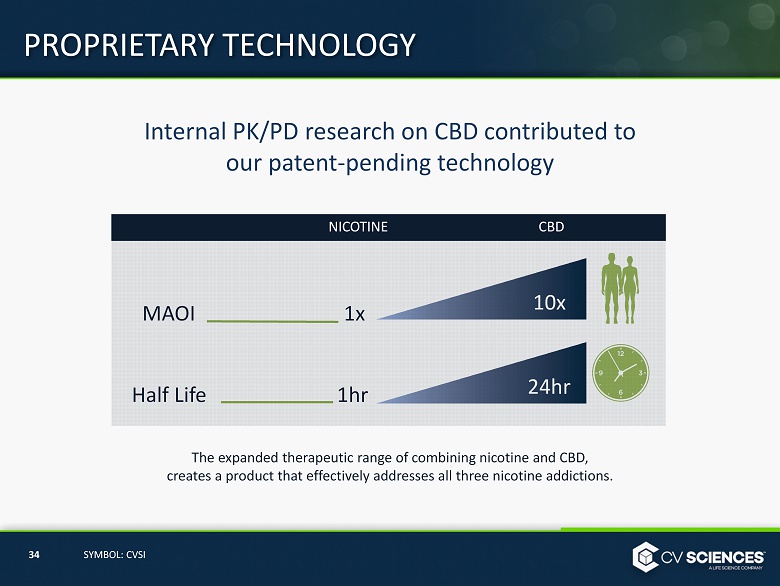
Internal PK/PD research on CBD contributed to our patent - pending technology The expanded therapeutic range of combining nicotine and CBD, creates a product that effectively addresses all three nicotine addictions. PROPRIETARY TECHNOLOGY 34 SYMBOL: CVSI NICOTINE CBD MAOI Half Life 1x 1hr 10x 24hr
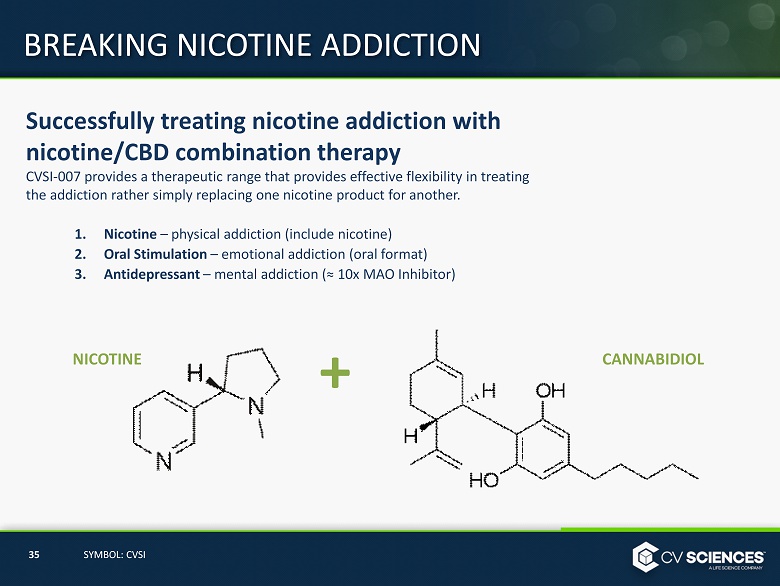
Successfully treating nicotine addiction with nicotine/CBD combination therapy CVSI - 007 provides a therapeutic range that provides effective flexibility in treating the addiction rather simply replacing one nicotine product for another. 1. Nicotine – physical addiction (include nicotine) 2. Oral Stimulation – emotional addiction (oral format) 3. Antidepressant – mental addiction (≈ 10x MAO Inhibitor) BREAKING NICOTINE ADDICTION NICOTINE CANNABIDIOL + 35 SYMBOL: CVSI

No current FDA - approved drugs (to treat smokeless tobacco use/addiction) OTC Treatments – Nicotine Replacement Therapies (NRTs) NRTs are the most widely used pharmacological product in treating nicotine addiction. Replacing cigarettes or smokeless tobacco products with another nicotine - only product, such as an NRT that is available OTC, has shown very high relapse rates. NICOTINE ADDICTION RELAPSE 36 SYMBOL: CVSI Studies have shown up to 93% of over - the - counter NRT users relapse and return to tobacco use within 6 months.
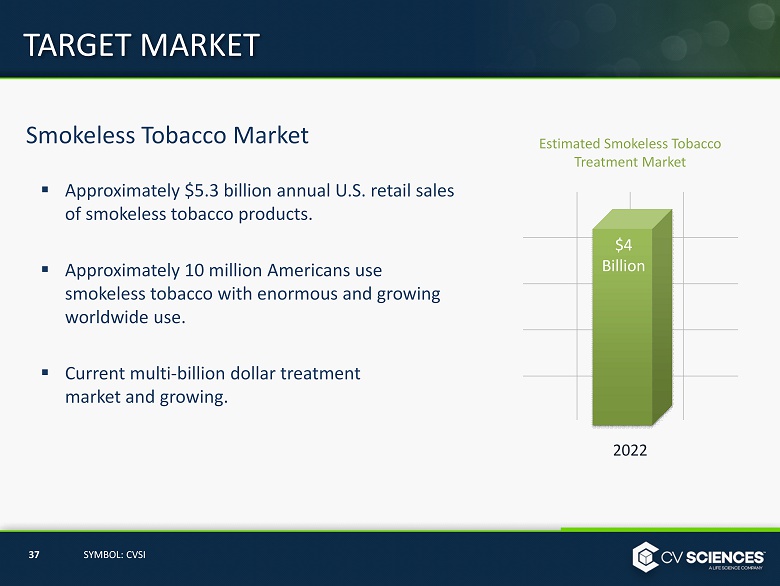
Smokeless Tobacco Market ▪ Approximately $5.3 billion annual U.S. retail sales of smokeless tobacco products. ▪ Approximately 10 million Americans use smokeless tobacco with enormous and growing worldwide use. ▪ Current multi - billion dollar treatment market and growing . TARGET MARKET 2022 Estimated Smokeless Tobacco Treatment Market $4 Billion 37 SYMBOL: CVSI

EPIDIOLEX APPROVAL 38 SYMBOL: CVSI EPIDIOLEX® (cannabidiol) oral solution ▪ First FDA approval of Cannabidiol

▪ Engaged with the FDA ▪ Complete nonclinical toxicology studies in 2018/2019 ▪ Submit Investigational New Drug (IND) application in early 2019 ▪ Begin clinical trials in 2019 DRUG DEVELOPMENT TIMELINE 39 SYMBOL: CVSI

FDA PRESS RELEASE – AUGUST 3, 2018 40 SYMBOL: CVSI Statement from FDA Commissioner Scott Gottlieb, M.D., on new steps the agency is taking to support the development of novel nicotine replacement drug therapies to help smokers quit cigarettes Posted on Aug 3, 2018 in FDA Press Releases As a public health agency, there is no greater impact we can have to improve the health of our nation than to significantly r edu ce the rate of tobacco - related disease and death. Through the U.S. Food and Drug Administration’s comprehensive framework for regulating nicotine an d t obacco, we’re developing policies that support the possibility of a world where combustible cigarettes could no longer create or sustain ad dic tion. A key part of this framework are steps to pave the way for products that help currently addicted smokers move away from the deadliest form of ni cot ine delivery. Part of this work requires that we recognize that nicotine, while highly addictive, is delivered through products posing a co nti nuum of risk – with combustible cigarettes at one end, to nicotine replacement therapy (NRT) products at the other. We’re working on multiple fro nts to recognize the role that more novel forms of nicotine delivery could play in achieving our public health goals, as part of an appropriately regul ate d marketplace. This not only includes encouraging innovation of potentially less harmful tobacco products for those adults who still seek to use nico tin e (such as e - cigarettes), but also taking a closer look at our overall approach to the development and regulation of NRT products that are regulated as dr ugs, and designed to safely reduce withdrawal symptoms, including nicotine craving, associated with quitting smoking. The development of novel NRT products, regulated as new drugs , is a critical part of our overall strategy on nicotine. We know that about 70 percent of adult smokers in the U.S. want to quit . In fact, nearly half try to quit each year. But few succeed. Use of FDA - approved NRT products is generally considered to double the likelihood of a successful quit attempt (with variations between pro ducts). Our ultimate goal is to help more smokers completely quit cigarettes.

1. Outstanding Financial and Operational performance 2. Strong Balance Sheet 3. These fundamentals are institutionally relevant 4. Potential uplisting to Nasdaq 5. Huge market expansion for Consumer Products - $1.6B by 2021 6. 2018 Farm Bill 7. EPIDIOLEX® approval 8. No FDA approved drugs for smokeless tobacco addiction 9. Smokeless tobacco cessation - $4 billion market POSITIVE FACTORS 41 SYMBOL: CVSI
CV Sciences, Inc. Joseph Dowling, CEO 866 - 290 - 2157 ir@cvsciences.com cvsciences.com Media & Investor Relations IRTH Communications Robert Haag, Managing Director 866 - 976 - 4754 ir@cvsciences.com CONTACT 42 SYMBOL: CVSI