
Exhibit 99.1

Drug Development Program Overview LDMicro Invitational Conference June 8, 2016

Safe Harbor and Disclaimer Safe Harbor: This presentation may contain certain forward - looking statements and information, as defined within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and is subject to the Safe Harbor created by those sections. This material contains statements about expected future events and/or financial results that are forward - looking in nature and subject to risks and uncertainties. Such forward - looking statements by definition involve risks and uncertainties . This Overview does not constitute an offer to sell or a solicitation of an offer to buy any securities of CV Sciences, Inc . The company is not soliciting any investment from this presentation or event attendees. Offers to sell or solicitations of offers to buy securities of the company will be made pursuant to the registration requirements of the Securities Act of 1933, or regulations of the Securities and Exchange Commission, and under relevant state securities laws; or in accordance with lawful exemptions from registration requirements under applicable federal and state securities laws and regulations . Disclaimer: Mr. Jonnie R. Williams Sr., is the named inventor of the CV Sciences Inc. (“CVSI ”) clinical drug candidate. Additionally, Mr. Williams is the named inventor of numerous patents and patent applications including many relating to tobacco. Mr. Williams has had significant experience in the tobacco industry and is a pioneer in the reduction of tobacco - specific nitrosamines. As a matter of disclaimer, Mr. Williams currently serves as a part - time paid consultant advising CVSI on an as - requested basis in connection with its Cannabidiol (“CBD“) drug development program. Mr. Williams is an independent contractor and does not serve as an employee, agent or member of CVSI management. Mr. Williams was a founder and senior officer of CanX, Inc., which CVSI acquired on December 30, 2015. 2

• Developing CBD based potential FDA approved drugs . • Manufacturing and marketing branded consumer products . Corporate Overview CV Sciences Inc . is a leader in marketed Cannabidiol (a) (CBD) products: 3 (a) Cannabidiol (or CBD) is a compound derived from Cannabis that is reported to potentially have health benefits. ClinicalTrials.gov currently lists 100 studies utilizing CBD or other cannabidnoids. 1

Drug Development Program - Highlights • Initial Drug Candidate - ( CVS I - 007 ) chewing gum combines CBD and Nicotine - Patent Pending . • Proposed Claims - To support cessation of smokeless tobacco use/addiction . • Target Market - Smokeless tobacco market consisting of approximate $5.3 b illion in annual sales to approximately 9 million consumers (US Retail) . 2 • Proposed FDA Regulatory Pathway - 505b - 2 . Overview of CBD Drug Development Program In December 2015 , CV Sciences acquired CanX Inc., a Pre - C linical drug development company focusing on significant unmet medical needs. 4

Drug Development Program - Unmet Need 5 NO drug is FDA approved to support cessation of smokeless tobacco use and addiction . Urgent Unmet Medical Need: • Smokeless tobacco is carcinogenic (risk of esophageal, oral and pancreatic cancers) . 3 • Smokeless tobacco is strongly addictive . • Smokeless tobacco is an epidemic as recognized by Surgeon General . • Serious impact on youth: 5.8% of white males 12 - 17 years of age reported use of smokeless tobacco . 4 • Approximately 250,000 deaths world wide each year attributed to s mokeless tobacco use . 5

Drug Development Program : Target Market 6 Smokeless Tobacco Market • Approximately $5.3 billion annual US retail sales of smokeless tobacco products. 6 • Approximately 1.3 billion units of smokeless tobacco products sold annually in US. 6 • Approximately 9 million Americans use smokeless tobacco with enormous and growing worldwide use. 7 • Approximately 32 percent of rural, white males 12 - 17 years of age are either experimenting with, or at - risk for, using smokeless tobacco. 8 • Smokeless tobacco is one of the most addictive and potent ways of consuming tobacco (holding an average - size dip in the mouth for just 30 minutes can deliver as much nicotine as smoking three cigarettes ). 9
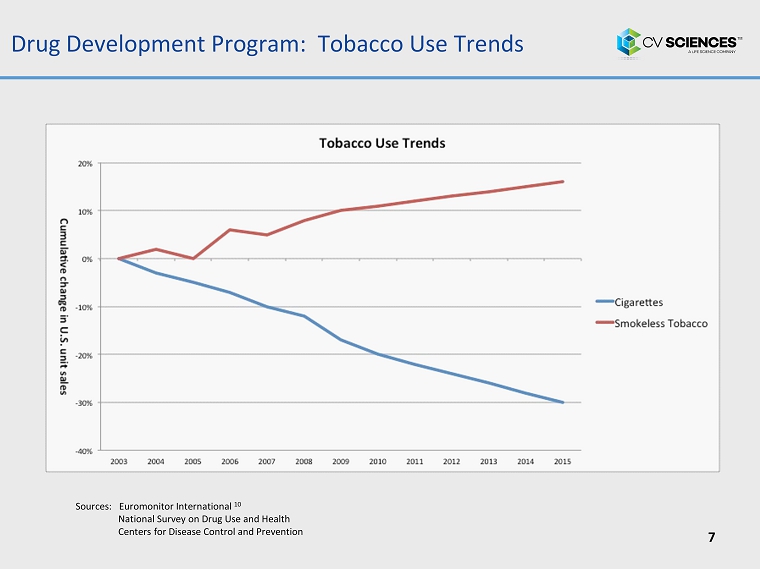
Drug Development Program : Tobacco Use Trends 7 Sources: Euromonitor International 10 National Survey on Drug Use and Health Centers for Disease Control and Prevention
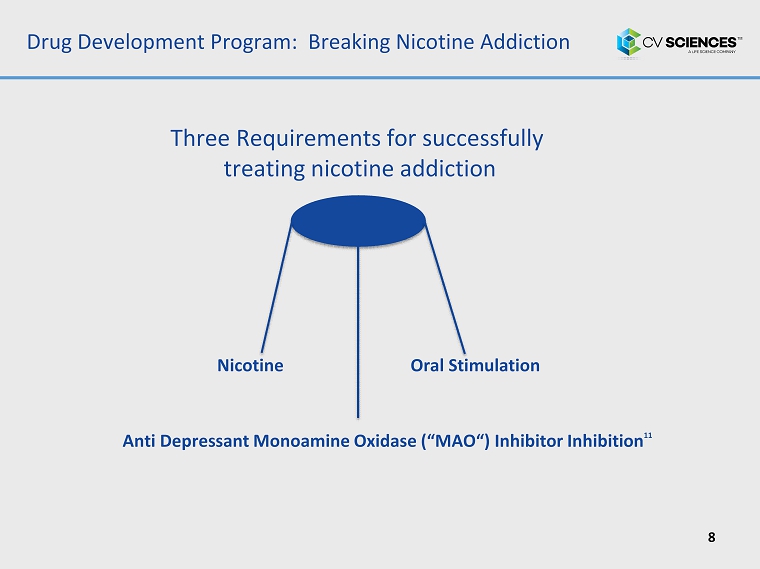
Drug Development Program: Breaking Nicotine Addiction 8 Nicotine Anti Depressant Monoamine Oxidase (“ MAO “) Inhibitor Inhibition 11 Oral Stimulation Three Requirements for successfully treating nicotine addiction

Drug Development Pr ogram: Initial Drug Candidate 9 The initial drug candidate ( CVS I - 007 ) is a chewing gum containing Nicotine and CBD to support cessation of smokeless tobacco use and addiction . 1. CBD: to inhibit MAO and provide anti - depressant effect to replace tobacco alkaloids in pre - clinical trials. 11 2. Nicotine: to address nicotine addiction and cravings . 3. Chewing Gum: to provide oral stimulation . How CVS I - 007 satisfies three requirements of Smokeless cessation:
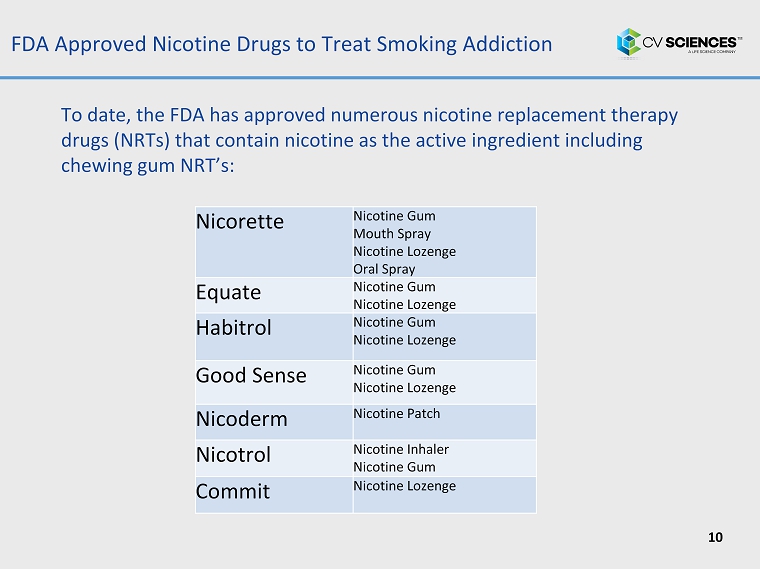
FDA Approved Nicotine Drugs to Treat Smoking Addiction 10 Nicorette Nicotine Gum Mouth Spray Nicotine Lozenge Oral Spray Equate Nicotine Gum Nicotine Lozenge Habitrol Nicotine Gum Nicotine Lozenge Good Sense Nicotine Gum Nicotine Lozenge Nicoderm Nicotine Patch Nicotrol Nicotine Inhaler Nicotine Gum Commit Nicotine Lozenge To date, the FDA has approved numerous nicotine replacement therapy drugs (NRTs) that contain nicotine as the active ingredient including chewing gum NRT’s:
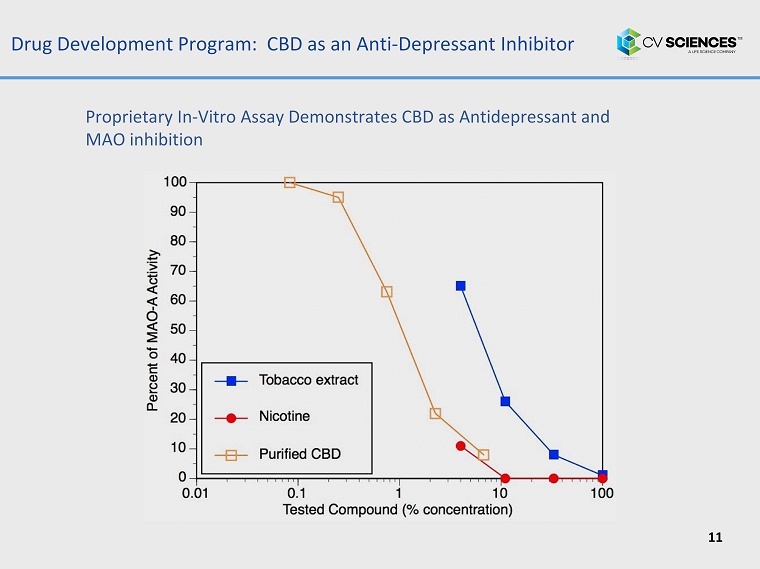
Drug Development Program: CBD as an Anti - Depressant Inhibitor 11 Proprietary In - Vitro Assay Demonstrates CBD as Antidepressant and MAO inhibition
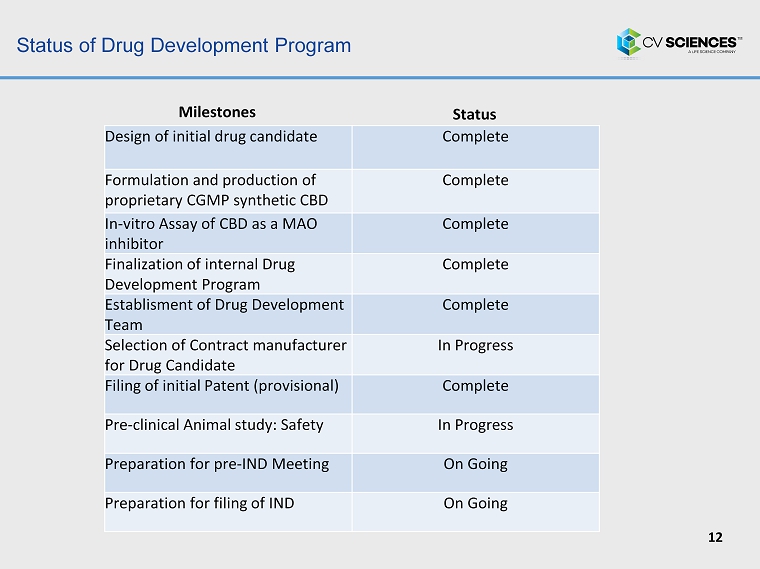
Status of Drug Development Program 12 Status Milestones Design of initial drug candidate Complete Formulation and production of proprietary CGMP synthetic CBD Complete In - vitro Assay of CBD as a MAO inhibitor Complete Finalization of internal Drug Development Program Complete Establisment of Drug Development Team Complete Selection of Contract manufacturer for Drug Candidate In Progress Filing of initial Patent (provisional) Complete Pre - c linical Animal study: Safety In Progress Preparation for pre - IND Meeting On Going Preparation for filing of IND On Going

Status of FDA approval of CBD as an Active Drug Ingredient Current regulatory environment for CBD as an FDA approved drug: • FDA Phase 3 human clinical trials of CBD are ongoing . • GW Pharma is seeking FDA approval for a CBD based drug based on a completed Phase 3 clinical trial . 12 • FDA approval for a CBD drug has received encouragement from the White House and the U.S. Senate (International Caucus on Narcotics Control ). 13 13

Drug Development Team 14 CV Sciences has assembled a qualified and experienced team to lead its drug development program. Chief Medical Officer - Dr. Chris Chapman - Dr. Chapman serves as Chairman and Chief Executive Officer of Chapman Pharmaceutical Consulting, Inc., which provides expert medical consultation on the development and management of domestic and global product development programs for biotech, pharmaceutical, and medical device products. He served as Senior Director of Medical Affairs with Quintiles/BRI, the largest contract research organization in the U.S., from 1995 unti l 2000. In that capacity, Dr. Chapman had oversight responsibility for the support of new drug applications, clinical studies, and device submissions to the FDA for approval. From 1992 until 1994, Dr. Chapman was Medical Director at Regeneron Pharmaceuticals. He currently serves as Chairman of the Chapman Pharmaceutical Health Foundation. Dr. Chapman is a graduate of the Georgetown University School of Medicine in Washington, D.C. Clinical and Regulatory Advisor - Ms. Shelly Goodman - Ms. Goodman is the CEO of The FourCe LLC and is the Head of Global Drug Safety and Pharmacovigilance for Portola Pharmaceuticals, Inc. She was previously Head of Global Drug Safety for both Cerexa Pharmaceuticals and Chiron Pharmaceuticals and has been a lead consultant to biotech, pharmaceutical and device companies for many years in project management, infrastructure, compliance and product development. She has designed and managed clinical development projects, created adverse event and labeling programs, prepared companies for successful pre - approval inspections, and has launched several products. She has worked in large hospitals, as well as startup through large global corporations, CRO’s, academic institutions and for the FDA.
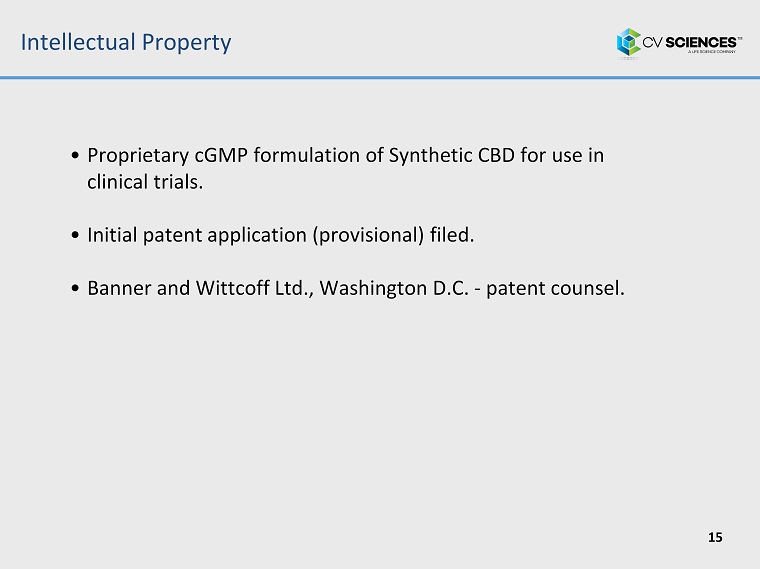
Intellectual Property 15 • Proprietary cGMP formulation of Synthetic CBD for use in clinical trials. • Initial patent application (provisional) filed. • Banner and Wittcoff Ltd., Washington D.C. - patent counsel.
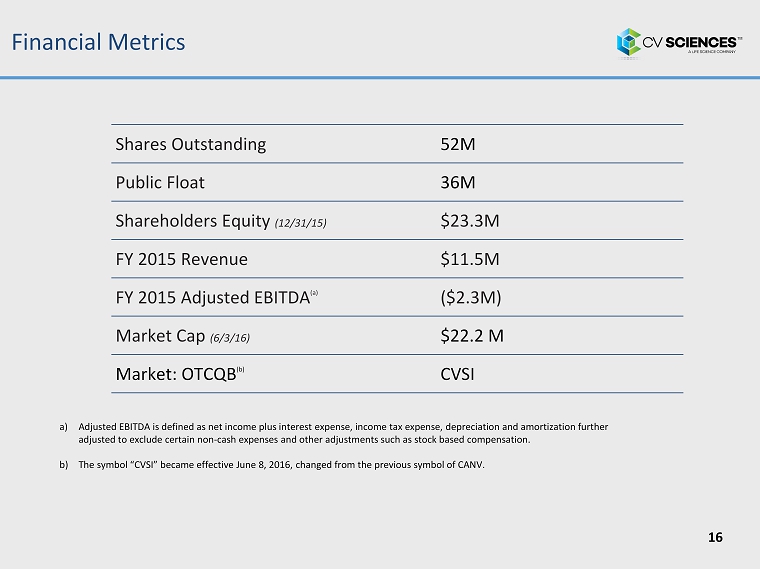
Financial Metrics Shares Outstanding 52M Public Float 36M Shareholders Equity (12/31/15) $23.3M FY 2015 Revenue $11.5M FY 2015 Adjusted EBITDA (a) ($ 2 . 3 M ) Market Cap ( 6 / 3 /16 ) $ 2 2 . 2 M Market: OTCQB (b) C VSI a) Adjusted EBITDA is defined as net income plus interest expense, income tax expense, depreciation and amortization further adjusted to exclude certain non - cash expenses and other adjustments such as stock based compensation. b) The symbol “CVSI” became effective June 8, 2016, changed from the previous symbol of CANV. 16

References References : 1. ClinicalTrials.gov, a service of the U.S. National Institutes of Health lists over 100 different studies utilizing Cannabidiol https://clinicaltrials.gov/ct2/results?term=cannabidiol&Search=Search 2. Statista, “Statistics and facts on smokeless tobacco in the U.S.”, http://www.statista.com/topics/2500/smokeless - tobacco - in - the - united - states/ 3. NIH National Cancer Institute, “Smokeless Tobacco and Cancer” http://www.cancer.gov/about - cancer/causes - prevention/risk/tobacco/smokeless - fact - sheet#q3 references the primary source International Agency for Research on Cancer. Smokeless Tobacco and Some Tobacco - Specific N - Nitros amines. Lyon, France: World Health Organization International Agency for Research on Cancer; 2007. IARC Monographs on the Evaluation of Carcinogeni c R isks to Humans Volume 89. https://monographs.iarc.fr/ENG/Monographs/vol89/mono89.pdf 4. 50 Years of Progress: A Report of the Surgeon General, 2014. Chapter 13, page 730. http://www.surgeongeneral.gov/library/reports/50 - years - of - progress/sgr50 - chap - 13.pdf 5. Dr. Mercola, "Smokeless Tobacco Kills More Than 250,000 Yearly, Worldwide" September 23, 2015 http://articles.mercola.com/sites/articles/archive/2015/09/23/smokeless - tobacco.aspx 6 . Statista, “Statistics and facts on smokeless tobacco in the U.S.”, http://www.statista.com/topics/2500/smokeless - tobacco - in - the - united - states/ 7 . National Cancer Institute Centers for Disease Control and Prevention U.S. Department of Health and Human Services, “Smokeless To bacco and Public Health", p.439. http://cancercontrol.cancer.gov/brp/tcrb/global - perspective/Chapter_15_SmokelessTobaccoAndPublicHealth.pdf 8 . The U.S. Food and Drug Administration, and agency of The U.S. Department of Health and Human Services, "FDA launches first ad ca mpaign focused on dangers of smokeless tobacco among rural teens" https://www.morningstar.com/news/pr - news - wire/PRNews_20160419DC75264/fda - launches - first - ad - campaign - focused - on - dangers - of - smokel ess - tobacco - among - rural - teens.print.html 9 . NIH Medline Plus, a publication of the National Institutes of Health and the Friends of the National Library of Medicine, "Se con dhand Smoke/"Light" Tobacco/ Smokeless Tobacco" https://www.nlm.nih.gov/medlineplus/magazine/issues/winter11/articles/winter11pg7.html 10. Adapted from "Smokeless Products Are Tough Test for Reynolds", The Wall Street Journal, March 26, 2010 http://www.wsj.com/articles/SB10001424052748703523204575129633103406778 Source: Euromonitor International 2009 data forecasts 11. Monoamine oxidases and tobacco smoking, Ivan Berlin and Robert M. Anthenelli , International Journal of Neuropsychopharmacology (2001), 4, 33 – 42 http://ijnp.oxfordjournals.org/content/ijnp/4/1/33.full.pdf 12. GW Pharmaceuticals, http://www.gwpharm.com/product - pipeline.aspx 13. http://www.feinstein.senate.gov/public/index.cfm/files/serve/?File_id=81b53476 - 64a3 - 4088 - 9bae - 254a84b95ddb U.S. Senate Caucus on International Narcotics Control, 112th Congress, 2nd Session, June 2012, "Reducing the U.S. Demand for Ill egal Drugs", page 15. The White House, Office of National Drug Control Policy, "Answers to Frequently Asked Questions about Marijuana," https://www.whitehouse.gov/ondcp/frequently - asked - questions - and - facts - about - marijuana#opposed 17

Contact Joseph Dowling, CFO Tel: 866 - 290 - 2157 joseph.dowling@cvsciences.com 2688 S. Rainbow Boulevard, Suite B Las Vegas, NV 89146 cvsciences.com 18